High School Chemistry Review
In the simplest form, a battery is an electrochemical device that stores and releases electrical energy through chemical reactions. A simple battery consists of three main components:
- Anode - the negative electrode
- Cathode - the positive electrode
- Electrolyte - conductive material that allows ions to flow between the electrodes
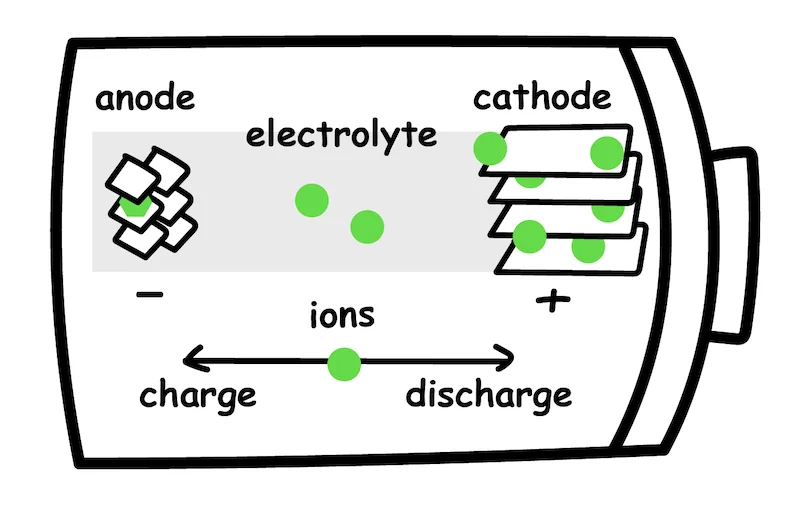
The battery is charged or used by moving ions from one side of the battery to the other. How does this work?
Imagine we have two tanks of water connected with a tube, and we fill both to the same level. Provided that both tanks have the same amount of water, no fluid will flow between the two tanks, since there is no pressure to do so.

However, when we start to add water to one tank, water will naturally flow into the other tank to reach a new equilibrium. In electronics, the difference in water level is called the potential difference.

When charging a battery, an external electrical current is applied to the battery, which creates a potential difference between the two electrodes. The potential difference is just like water pressure - it “pushes” ions, or charged particles, to move from one electrode to the other. When charging, these ions flow from the cathode to the anode. However, if we reverse the flow of ions, from the anode to the cathode, the battery will produce an electric current that can be used to power devices or allow mechanical work.
Why does my phone get warm?
If you’ve ever grabbed your smartphone during or immediately after a charge, you’ve probably felt that it’s warm. The heat that you’re feeling is coming from the battery, which heats up during use and charging. When a chemical reaction occurs in a battery the transfer of ions leads to energy being released or absorbed in the form of heat.
There are two sorts of reactions when it comes to heat: exothermic reactions, which release heat into the environment, and endothermic reactions, which absorb heat. When you pour water into an ice tray to make ice cubes, the freezing process is an exothermic reaction, since heat is lost by the water and released into the surroundings (i.e. your freezer). On the other hand, when you melt an ice cube, you need to add heat to the ice to turn it back into water, so this is endothermic.

Discharging, or using the battery, results in the release of energy, meaning these reactions are generally exothermic. Similarly, charging a battery results in the absorption of heat, an endothermic process.
The heat generated by a battery is a function of the current that flows through the battery (amps), and the internal resistance, which is a material property (we talk about it more here). The specific amount of heat generated or absorbed during reactions will depend on several factors, including the specific chemistry of the battery, the rate of charging or discharging, the ambient temperature, and the state of charge of the battery.
Let’s pause for a second to talk about how the ambient temperature affects reactions. If you’ve ever taken a physical chemistry class, you may remember the Arrhenius equation, which states that the rate of a chemical reaction increases exponentially as a function of temperature. For instance, the chemical reaction that breaks up 2 NO2 molecules into 2NO and one O2 is heat dependent, as shown below.

Why does this happen?
There is a minimum energy threshold needed for any reaction to take place. At higher temperatures, individual particles move faster, or have higher kinetic energy. This additional kinetic energy increases the number of molecules that meet the minimum energy threshold. In a battery, this means that it takes less additional energy to charge, or that you can discharge (e.g. turn an electric motor to move an EV) with additional power. Temperatures below the ideal operating temperature results in less kinetic energy, slower reaction times, and decreased power output. Similarly, temperatures above ideal operating temperature may lead to early degradation.
Batteries are a lot like people - always aging. In people, being alive produces side reactions, such as oxidation and cell death, that make us older. We can do things to reduce these effects, like exercise and sleep, or things that accelerate them, such as smoking and tanning. The same is true of batteries. When it’s hot enough, the extra energy in the battery can accelerate unwanted chemical reactions that age the battery prematurely. Thus, heat may cause loss of electrolyte, permanent damage, or even battery failure.
.webp)
Given the risks of excess heat in a battery, thermal management systems are essential to maintain safe operating temperatures. If the battery overheats, it can lead to safety hazards, reduced battery performance, and accelerated degradation of the battery components. Proper thermal control is key for optimal battery performance and extending battery life to recommendations or beyond. Although it does use some battery charge, aka range, to maintain the battery temperature, it’s well worth it for long lasting packs.
Effect of Heat on Batteries

Batteries at Rest
Even when you’re not driving your electric car, heat can have a significant impact on the battery. To decrease the negative effects of heat on EV batteries at rest, it is ideal to park in shaded areas or garages whenever it’s especially hot or sunny.
In addition to reducing direct heat exposure, keeping an EV plugged in (but not necessarily charging) will allow the BMS to cool the battery, ensuring there is external energy to maintain optimal battery temperature. Here are some ways heat can impact EV batteries at rest:
- Self-discharge: All batteries experience self-discharge over time. This is the gradual loss of charge even when the battery is not actively being used. Higher temperatures can accelerate the rate of self-discharge, causing the battery to lose its charge more quickly when parked in hot environments.
- Loss of capacity: Capacity loss is when a battery can store less energy over time. It’s what you experience with an older cell phone that is unable to stay charged for as long as when it was new. Prolonged exposure to high temperatures, especially when the battery is at a high state of charge, can lead to capacity loss over time. It will eventually lead to lower available range when the vehicle is in use.
- Lifespan: As discussed above, high temperatures accelerate the rate of chemical reactions within the battery, leading to faster degradation of active materials and reducing the number of charge and discharge cycles that a battery can undergo.
- Cell balancing: In multi-cell battery packs, like the ones in EVs, individual cells might have slightly different capacities or voltages. During rest periods, when the battery is not being actively balanced through charging or discharging, heat can exacerbate imbalances between cells, potentially affecting the overall performance and lifespan of the battery pack. Unbalanced cells can also affect how well the battery management system can evaluate range and state of charge.

- Thermal runaway: Although extremely rare, if the battery pack is exposed to extremely high temperatures or malfunctions, such as in a crash, it can lead to thermal runaway. This is a rapid, uncontrollable increase in temperature, ultimately resulting in fire or explosion.
Batteries In Use
Using any battery will produce heat, even though the heat produced by an EV is much less than the heat produced by a gas engine. It’s a natural byproduct of the chemical reactions. Although heat is unavoidable, there are some ways to reduce excess heat within the battery.
- Preconditioning: Modern EVs have the ability to precondition, which involves remotely activating the vehicle’s climate control system prior to driving. You can do this while the vehicle is plugged in, often via an app, allowing an external energy source to prepare the battery without generating excess heat.
- Avoid extreme temperatures: Ideally, avoiding use during the peak temperature hours is ideal. This means the planner trips for cooler times, such as the morning or evening. This is not always possible, and you should not inconvenience yourself for the marginal effect it has to drive in the heat.
- Driving style: Driving at high speeds generates more heat in the battery due to increased power demands. Reducing overall speed can help reduce heat buildup and minimize the stress on the battery. Additionally, gradual acceleration and deceleration will reduce the strain on the battery and help manage temperature fluctuations more effectively.
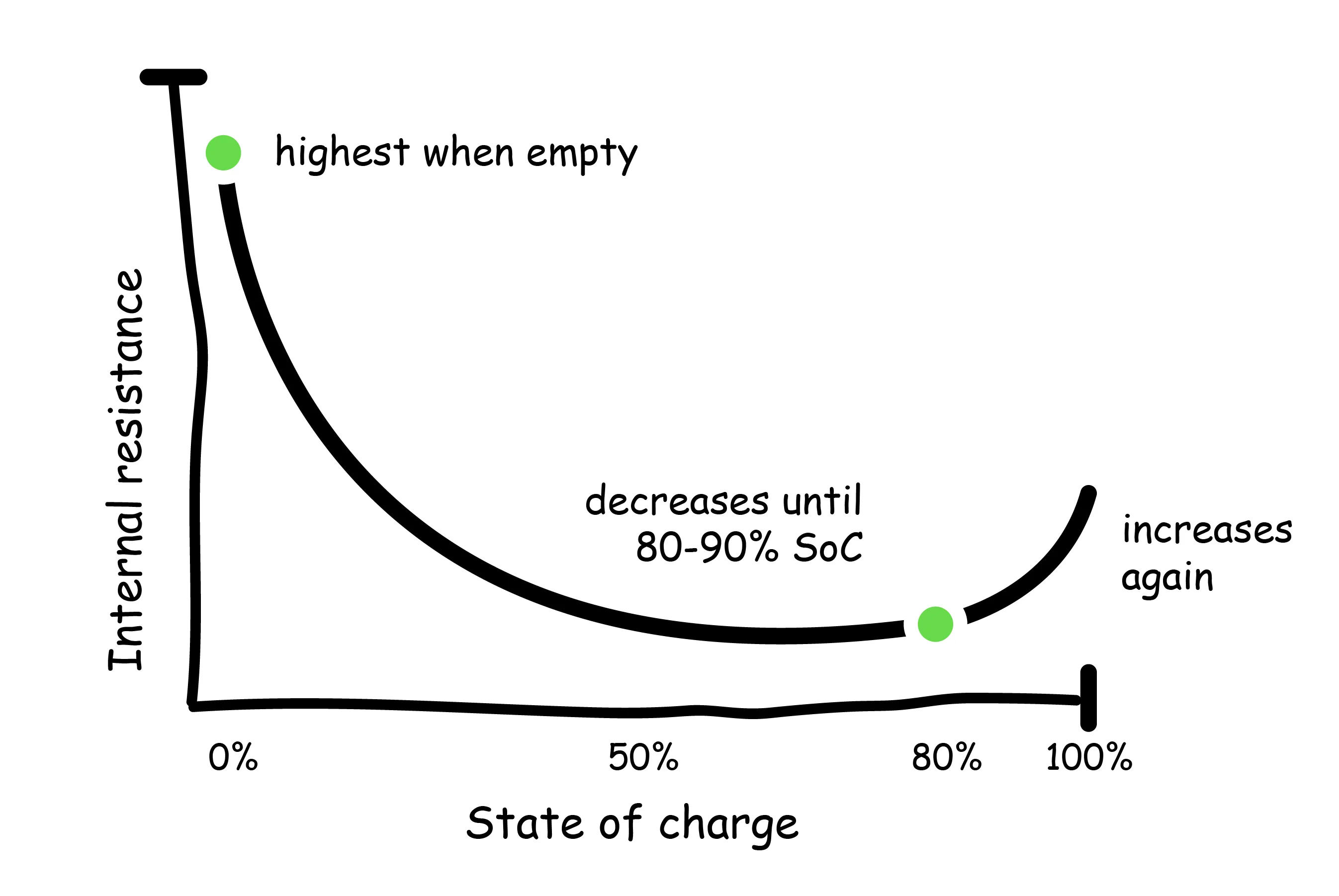
- State of charge (SoC): Higher states of charge (and very low states of charge) are associated with increased internal resistance within the battery. As discussed above, the heat generation is a function of the current and internal resistance. Therefore, at higher levels of charge, excess heat production within the battery is more likely to occur. Ideally, do not charge above 80% unless necessary.
Batteries Being Charged
Similar to battery discharge (i.e. being used), charging the battery results in heat production. Reducing the impact of excess heat on the battery while charging is the main step you can take to preserve battery health, charging efficiency, and safety.
- Preconditioning: Prepping the car for charging, or any use, will ensure that the battery starts the charging process at a lower temperature. In many modern EVs, if you set the car’s navigation to a DC fast charger, the thermal management system will automatically cool the battery in preparation.
- External conditions: If possible, charging in a shaded area or a garage, especially in hot weather, is ideal. Additionally, planning your charging sessions during cooler times of the day, such as early mornings or evenings, can help reduce the amount of heat exposure on the battery.
- Charging speed: Increased charging speeds result in more heat generation. This means avoiding fast charging, or, if not possible, at least avoiding fast charging under extreme conditions (e.g. direct sunlight, peak temperatures). Furthermore, some EVs allow the user to adjust charging current. Choosing a lower current will reduce the production of heat.



